前胸壁切除术可能会显著改变呼吸生理学,具体取决于是否累及整个胸骨、缺损的大小和重建类型。1,2根据仿生学原理,必须进行细致的术前计划,以进行根治性切除和重建。1为此,计算机断层扫描图像重建和数字减法正日益成为可视化胸壁受累程度的强制性辅助手段,并特别注意肿瘤边缘。为了完成浸润胸壁全层肿瘤的术前评估,对胸壁内侧的视频辅助胸腔镜评估可能有用,特别是当要评估乳腺癌或化疗后残余肿瘤的胸壁复发时。由于冷冻切片在骨标本上不可行,因此应获得高达 4 至 5 cm 的无肿瘤边缘。1虽然关于重建策略的决定应该是个体化的,但众所周知,仅切除一根肋骨所导致的缺陷可能不需要重建。然而,如果存在持续的肺疝风险,则特定患者的前单肋缺损(即肋骨 4 或 5)可能需要覆盖(即年轻运动员)。1对于恶性原发性胸壁肿瘤,应切除紧邻的肋骨,使其在无肿瘤边缘上切除相应长度的浸润段。1当涉及手镯和一个或两个锁骨时,这些锁骨可以切除而无需随后的重建,从而产生可接受的肩胛带运动。1用于胸壁重建的历史悠久的材料(即聚丙烯,聚乳糖素网状物或甲基甲基丙烯酸甲酯三明治[MMM]以及聚四氟乙烯[PTFE]贴片)仍然是有价值的选择,尽管新材料(即钛板,无细胞胶原基质网和冷冻保存的同种移植物)由于对感染的弹性和随时掺入宿主组织而越来越受欢迎。2,3
切除和重建技术
部分厚度—切除
皮肤切除术应保持在距宏观可见肿瘤边缘的安全边缘(2-3厘米)。当肿瘤溃疡时,皮肤切口应与发炎区域保持相同的距离。在任何情况下,对随后重建的关切都不应影响关于切除多少的决定。肌肉层沿着一条远离肿瘤的斜线分开,使整体标本类似于截断的锥体(图1A;视频 1)。如果可能,应避免皮下脂肪组织作为后续原发性或继发性闭合的加固。解剖从疤痕未参与的肋骨的骨膜开始。在多次局部复发或先前辐射区域的情况下,肋骨平面被粘附以抬高上覆(胸)肌肉(图1B;视频 1)。此后,标本被悬停在尾颅上,并横向和内侧分开,以识别和分割主要血管供应。

部分厚度-重建
在直接的情况下,可以通过皮下破坏伤口边缘或使用皮肤近似或皮肤移植物的整形手术技术来实现一级闭合。1,2相当大的缺损覆盖有旋转、转位和插入肌皮瓣。2我更喜欢V-Y推进皮瓣,它使外科医生能够覆盖广泛的前外侧缺损,并允许对供体伤口边缘进行充分的近似。4基于升高的背阔肌的三角形皮肤岛向前旋转(图2A;视频 1)。4使用聚乳糖素 2-0 或 3-0 缝合线(即,背阔肌边缘到接受者区域的周围肌肉)进行层对层缝合通常足以将皮瓣固定到位,而无需将其固定到下面的肋骨上。4或者,当皮肤幸免于允许部分增厚缺陷的初级闭合时,背阔肌皮瓣可以升高并前旋转(图2B;视频 1)。毋庸置疑,这些肌肉或肌皮瓣也可以转置以覆盖全层缺陷。

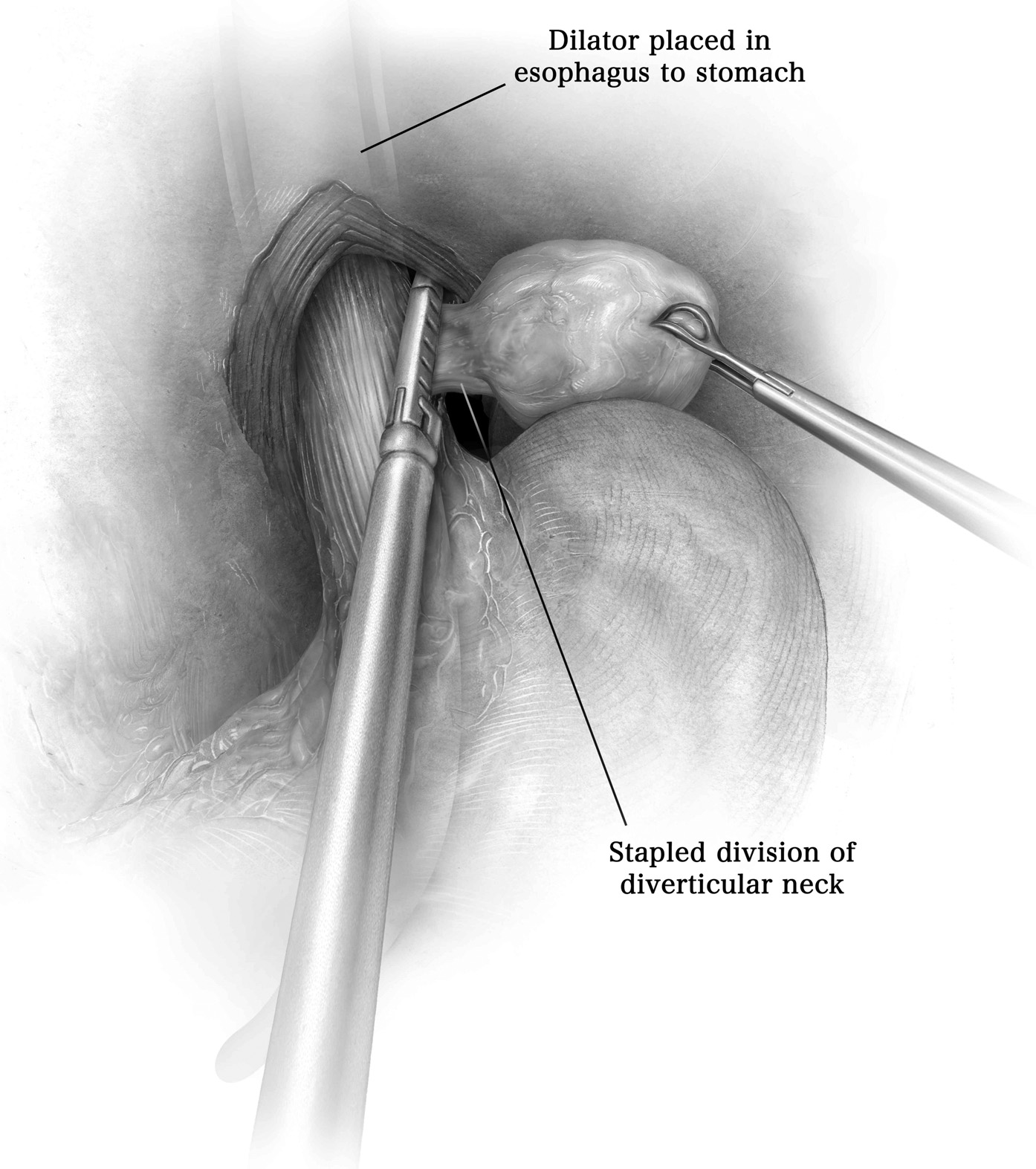
全厚度—切除
最上层肋间空间通过电烙术打开,使其与要移除的最高肋骨的上缘接触(图3;视频 1)。一旦确定了要切除的最低肋骨,沿着紧挨着的下肋骨的上缘通过电烙术打开肋间空间。手指触诊或胸腔镜下胸壁内壁探索可能有助于切除边缘的定义。肋间血管结扎并分开在受累一根肋骨或肋骨的上方和下方(图3;视频 1)。通过进行前后肋骨切开术,切除足够长度的骨段以确保无肿瘤边缘以及相应的肋间肌肉(图3;视频 1)。

Full Thickness—Reconstruction
As a rule, a material with enough rigidity to ensure chest wall stability needs to be selected. In this setting, PTFE and MMM have proved to serve this purpose.5, 6 The latter are often used in the event of sternal replacement.1, 2, 6 However, when PTFE is used for extensive anterior chest wall defects requiring rib resection up to the entire lateral aspect of the sternum, the reconstruction will be based on a series of sternal punches going through the sternum to accommodate the anchoring sutures for either the PTFE or the MMM4, 5, 6 (Video 1). However, titanium plates in association with acellular collagen matrix or cryopreserved homografts could be a viable alternative for reoperations and heavily irradiated areas7 (Fig. 4B, C; Video 1). Titanium plates can be molded to fit the rib contour and then cut to the desired length (Fig. 4C; Video 1). The plates are anchored to the bony ribs with titanium screws of increasing length as required by the rib thickness. Usually, 3-4 screws on each side represent sufficient warranty against fracture or dislocation, which in our experience occurred in only one patient, who required plate replacement with acellular collagen matrix patching. Bone drills are of utmost value to guide the precise insertion of the screw (Fig. 4C; Video 1).7 Usually, titanium plates are placed with a 2:1 ratio compared to the number of resected ribs. If an acellular collagen matrix prosthesis is selected, the sternal Sweet puncher or a bone drill can be used to facilitate placement of the anchoring sutures (2-0, non-reabsorbable) around the perimeter of the prosthesis (Fig. 4B; Video 1).7 A combination of titanium plates and acellular collagen matrix patch can be used; in this case, the central prosthesis is sutured to the plates to confer additional stability7 (Fig. 4C). Alternatively, the titanium plates are applied without internal coverage material. If the wound cutaneous layer can be closed primarily, the titanium plates are covered with polyglactin meshes to avoid friction on the overlying skin (Fig. 4D; Video 1).

Full Thickness—Resection (Sternum)
If the sternum needs to be removed, the division of the bony rib starts laterally on the side opposite to the most severely involved by the sternal neoplasm. The width of lateral extension is dictated by the need for safe, tumor-free margins.1, 2, 7 If the tumor infiltrates the body of the sternum symmetrically, the resection is started on the left side and then carried out counterclockwise to the level of the same contralateral rib (in a “U” fashion). In any case, the chondrocostal release is performed up to at least one intercostal space superior to the macroscopic appearance of the tumor.7 The mammary vessels are routinely identified and divided. The intercostal pedicle is usually dissected through an extraperiosteal route. However, if the primary tumor is a chondrosarcoma, the periosteum should be included in the specimen because its lymphatics may represent a possible route for local recurrence and metastatic dissemination. As the separation of the sternum from the ribs continues, the specimen is suspended, thereby allowing for visualization of the mediastinum. The adhesions connecting the endothoracic fascia to the pericardial fat are divided. If the manubrium can be spared, it is prepared by blunt dissection and divided with a sternal saw immediately above the second chondrosternal joint. If the tumor involves the upper sternum, the sternotomy incision is carried laterally onto the clavicles configuring a T-shaped incision (Fig. 5A).8 The clavicles are dissected in a subperiosteal plane to avoid injury of the adjacent vessels.8 Finger dissection is needed to break retrosternal ligaments behind the manubrium. The mammary pedicles are usually divided at this point and the clavicles are resected as needed en bloc with the manubrium8 (Fig. 5B).

Full Thickness—Reconstruction (Sternum)
Variable options are available for poststernectomy stabilization.2, 3, 5, 6 The selection of the reconstructive material depends on the size of the defect, on previous irradiation or surgery, on concurrent infection, and on whether the manubrium is preserved.1, 2 If the sternum is completely removed up to the clavicles, anterior chest wall stabilization can be achieved by means of PTFE or MMM prostheses. If the manubrium is spared, a neosternum is created by fitting a cryopreserved homograft, (ie, iliac crest7; Fig. 6A). The neosternum is locked into the manubrium with titanium screws.7 A drill or a sternal puncher is used to accommodate the non-reabsorbable sutures (2-0 polypropylene) anchoring the MMM sandwiches to the neosternum (Fig. 6A, B).7 Other cryopreserved homografts can be used (ie, ribs; Video 1) to bridge the defect after sternectomy. Cryopreserved ribs are fixed to native lateral rib segments through either Kirschner wires or titanium plates. Cryopreserved homografts may require an omental, vascularized bed to facilitate vascular inosculation and progressive incorporation into the host. The same technique is used to protect the mediastinum when titanium plates are selected to bridge the poststernectomy defect (Fig. 6C; Video 1).8 The omentum is preferably raised through an upper midline limited laparotomy and passed under and over the homografts or the titanium plates; alternatively, the omental flap can be split in 2 different layers (Fig. 7A, B; Video 1).8 Omental flaps are secured to surrounding structures with polypropylene 2-0 sutures. A potential alternative to omental flaps is represented by the combination of titanium plates and acellular collagen matrix.1, 3 If the defect is in the lower sternum, rigid stabilization may not be necessary. In this case, it might be sufficient to raise the pectoralis major muscles bilaterally and approximate them on the central line, thus covering the defect with viable tissue (Fig. 8).


结论
如今,由于为随后的重建引入了改进的材料,因此可以对前胸壁肿瘤的原发性和重切除进行多功能,个体化的方法是可能的。1一丝不苟的技术和对结构和功能仿生概念的坚持代表了实现最佳结果的基本原则。1在这种情况下,尽管复杂患者提供的困难越来越大,但术后发病率正在逐渐下降。1然而,在为重建选择最适当的材料时,必须考虑到经济问题。1,3在这种情况下,当既不对先前照射的手术部位也不对感染的手术部位进行一级切除术时,仍应使用历史悠久的假体。1,3,9,10相反,根据复发性肿瘤的扩展,重做手术通常需要材料的组合。1,3,4因此,越来越多的胸壁前壁切除术后重建选择。此外,在进行复杂的重建时,应寻求多学科专门知识。1此外,胸腔镜引导胸壁切除术可以进一步扩大手术器械库。1因此,以类似于肺癌管理的生物分子革命的方式,可以为胸壁前壁肿瘤患者计划"靶向"切除和重建。
原文链接:http://www.xxwk.net/archives/2045