Background/Aim
Vein-first lobectomy (VFL) in lung cancer might reduce shedding of circulating tumour cells (CTCs). This study assessed the clinical significance of VFL.
Patients and Methods
Lung cancer patients undergoing lobectomy and CTC testing were evaluated. The primary evaluation item was postoperative clustered CTC detection, and the secondary outcome measures were the 2-year overall survival and recurrence-free survival rates according to the status of VFL and postoperative clustered CTC.
Results
Eighty-six patients with similar backgrounds, except for lobe resection and pulmonary vein dissection time, showed postoperative clustered CTC identification rates of 43.8% and 37.9% in the VFL group (n=57) and no-VFL group (n=29), respectively. However, prognosis was not significantly different, although the presence of clustered CTC after surgery was a predictor of recurrence.
Conclusion
The status of postoperative clustered CTC was similar regardless of VFL or not, although the detection of clustered CTC was a predictor of recurrence.Keywords: Vein-first dissection lobectomy, circulating tumour cell, recurrence-free survival, overall survival, no-touch isolation technique
Lung cancer is one of the leading causes of cancer death (1); thus, we need to increase and to control the accuracy of lung cancer treatment. Lung cancer can recur despite complete resection (2), and some of these recurrences are attributed to residual tumour cells after surgery (3). Circulating tumour cells (CTCs) are tumour cells found in the peripheral circulating blood, of which clustered CTCs has high potential to develop into recurrent tumours (4). Thus, decreasing the number of shed CTCs will reduce the risk of metastasis. Since the formation of the metastatic lesion occurs via clustered CTC rather than through single CTC (4,5), it is possible to control recurrence if a strategy targets clustered CTCs.
In lung cancer surgery, the amount of postoperatively shed CTCs might be reduced via vein-first dissecting lobectomy (VFL). VFL has been reported to be associated with good prognosis after pulmonary lobectomy for lung cancer (6-9). In contrast, several studies have found that VFL has limited therapeutic benefits for reducing recurrence (10-13). A recent prospective randomised study (6) has revealed that among lung cancer patients undergoing lobectomy, the postoperative increase in CTC count was more limited in the VFL group than in the no-VFL group. Furthermore, a retrospective propensity score-matching analysis has revealed superior survival in the VFL group than in the no-VFL group (6). However, in that study, the CTC counting was not performed using the morphological method; thus, the status of clustered CTC cannot be assessed (14).
In practice, the presence of clustered CTCs is a significantly reliable predictor of lung cancer existence (15) and postoperative recurrence (16). Since haemostatic metastasis has been proven to occur primarily by clustered CTCs (4,5), assessing the perioperative clustered CTC status and the prognosis of VFL and no-VFL cases will be useful in understanding the clinical implications of VFL for lung cancer. This study aimed to investigate the clinical significance and prognostic impact of VFL mainly according to changes in the clustered CTC status in the perioperative period and to assess the secondary outcome measures according to prognosis.Go to:
Patients and Methods
Study design and patient selection. This study was approved by the institutional review boards of Hoshigaoka Medical Center (HMC) (No.1718) and Nara Medical University Hospital (NMUH) (No.1412). Informed consent was obtained from all participants in the study.
Patients with pulmonary nodule with final diagnoses of lung cancer were evaluated in a retrospective, observational manner. We enrolled and evaluated patients who underwent lobectomy and CTC testing before and immediately after the completion of lobectomy. The primary outcome measure was the detection rate of postoperative clustered CTC according to the status of VFL or no-VFL. The secondary outcome measures were the 2-year overall survival (OS) and recurrence-free survival rates (RFS) according to the status of VFL and postoperative CTC.
Definition of VFL. VFL was defined as a procedure in which pulmonary venous dissection is completed before any part of pulmonary artery dissection through the incision of the mediastinal pleura. In addition, even before any part of pulmonary artery dissection, the complete pulmonary vein dissection after the opening of the inter- lobular pleura was not classified as VFL.
Surgical methods. All patients underwent routine enhanced thin section (1.0 mm width) computed tomography (CT) to obtain multi-planar reconstruction (axial, coronal, and sagittal shadow), on where we assessed the method of surgery.
Thoracic surgery was performed under general anaesthesia and with a double-lumen endotracheal tube. The patient was placed in a lateral position with the ipsilateral upper extremity flexed and fixed onto the hand shelf. The surgeon stood on the left side of the patient. An access port was made in the 7th or 8th intercostal space at the mid-axillary line. A utility incision (4-5 cm) was made at the posterior axillary line in the 4th or 5th intercostal space, and another one (1-2 cm) was made at the anterior axillary line in the 4th or 5th intercostal space. These ports allowed the concomitant use of two instruments. In addition, an assistant port (0.6 cm) was used at the posterior axillary line in the7th or 8th intercostal space if needed.
Detection of circulating tumour cells. Peripheral arterial blood (3 ml) was collected inside the operating room immediately before surgery and placed in an ethylenediaminetetraacetic acid tube. When lung cancer was pathologically confirmed, another blood sample was obtained after completion of pulmonary resection. All samples were processed within 4 h from the end of the operation. The presence of CTCs in the blood samples was evaluated using the ScreenCell® CTC selection kit (ScreenCell Westford, MA, USA), which uses a size-based selection method (17). The extracted cells were then stained with haematoxylin and eosin and observed under a light microscope.
The presence of CTCs was determined based on a cytology atlas for CTCs from solid cancers (18). Suspicious cells were not considered CTCs in the present study. The CTC detection results were divided into three types based on the morphological findings: no CTCs detected (N), only single CTCs were detected (S), and CTC clustereds were detected (C). A “clustered” was defined as ≥4 cells to minimise the contribution of collection- or preparation-related artifacts. All CTC evaluations were performed by a surgeon (NS), and the diagnoses were confirmed by pathologists (IT or CO).
Data collection and follow-up. Data were collected from the medical records, and videos of the surgery were also collected by one investigator (NS). Mortality and recurrence data were collected by one investigator (NS). All patients were followed up at 1-month to 3-month intervals, and follow-up included physical and chest radiography examinations and blood testing for tumour markers. The patients also underwent thoracoabdominal CT scans at 6-month intervals. The patients were followed up for a median of 50 months (range=24-74 months), and the last follow-up examination was performed in January 2020. Recurrence was classified as none, local (i.e., resection margin recurrence), regional (i.e., ipsilateral intrathoracic recurrence), or distant (i.e., metastasis outside the ipsilateral intrathoracic cavity).
Statistical analyses. Inter-group comparisons were performed using the t-test in comparison of mean values and Fisher’s exact test in comparison of frequency. Multivariable logistic regression analysis of VFL in the end point of detecting CTC postoperatively, was performed with covariates of tumour vessel invasion, types of CT appearance (solid or not), and size of invasion. Survival was evaluated based on Kaplan–Meier curves and compared using the log-rank test. All statistical analyses were performed using the freely available “EZR” software (19), which is based on R and R commander. Differences were considered statistically significant at p-values <0.05.Go to:
Results
Patient characteristics and circulating tumour cell status. Among the 302 patients with pulmonary nodule who underwent surgery at HMC or NMU between June 2014 and June 2018, we enrolled 86 patients who underwent lobectomy for lung cancer and CTC testing before and immediately after the completion of lobectomy.
The characteristics of the overall cohort and of the VFL groups are shown in Table I. The most frequently involved lobe was the right upper lobe (34.9%), followed by the right lower lobe (29.1%), left upper lobe (18.6%), left lower lobe (14.0%), and right middle lobe (3.5%). Most of the lesions were pure solid lesions on CT (82.6%), and the most common pathological diagnosis was invasive adenocarcinoma or squamous cell carcinoma (81.5%). Other histological diagnoses were pleomorphic carcinoma (n=5), large cell neuroendocrine carcinoma (n=4), not otherwise specified (n=3), adenosquamous cell carcinoma (n=2), and carcinoid (n=1). Overall, 25% of the patients had pathological stage >I disease, and 33% received adjuvant treatment (oral tegafur/uracil or intravenous carboplatin plus paclitaxel). There were significant differences in prevalence of lobe containing tumour and time of completion of pulmonary vein dissection between the VFL and no-VFL groups, although other variables were not significantly different.
Table I
Clinicopathological patient characteristics.
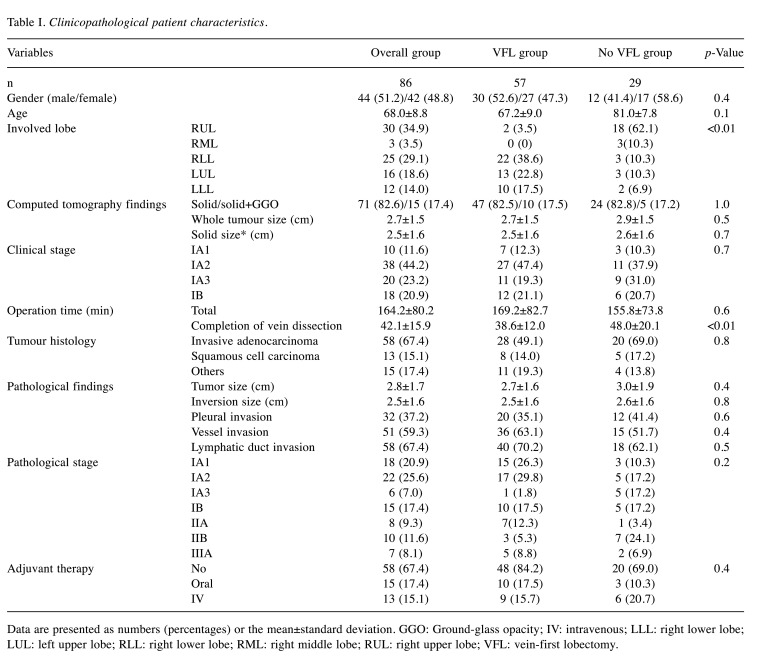
Data are presented as numbers (percentages) or the mean±standard deviation. GGO: Ground-glass opacity; IV: intravenous; LLL: right lower lobe; LUL: left upper lobe; RLL: right lower lobe; RML: right middle lobe; RUL: right upper lobe; VFL: vein-first lobectomy.
The CTC status in the overall cohort and in the VFL and no-VFL groups is shown in Table II. The preoperative CTC detection rate was 39.5%, and the CTC count was 1.6±3.1 [mean±standard deviation (SD)]. The CTC detection results were N, S, and C in 65.1%, 12.8%, and 22.1% of the patients, respectively, and there were no significant differences between the VFL and no-VFL groups. Postoperatively, the CTC detection rate was 57.0%, and the CTC count was 3.0±3.8 (mean±SD). There were 43.0%, 15.1%, and 41.9% of the patients who had N, S, and C CTC results, respectively, and there were also no significant differences between the VFL and no VFL groups. In addition, the change in CTC status beyond surgery was also not significantly different between the two groups (Table III).
Table II
Preoperative and postoperative CTC detection status according to VFL or no VFL.
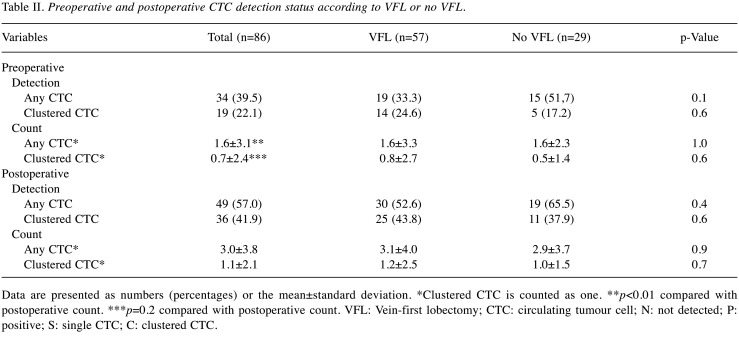
Data are presented as numbers (percentages) or the mean±standard deviation. *Clustered CTC is counted as one. **p<0.01 compared with postoperative count. ***p=0.2 compared with postoperative count. VFL: Vein-first lobectomy; CTC: circulating tumour cell; N: not detected; P: positive; S: single CTC; C: clustered CTC.
Table III
Change of CTC status following lobectomy according to VFL or no-VFL.
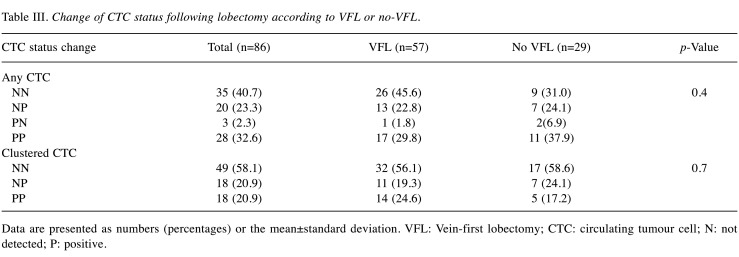
Data are presented as numbers (percentages) or the mean±standard deviation. VFL: Vein-first lobectomy; CTC: circulating tumour cell; N: not detected; P: positive.
In multivariate logistic regression analysis including VFL, tumour vessel invasion, types of CT appearance (solid or not), and size of invasion as covariates, it was revealed that only tumour vessel invasion alone was independent, when the end point was set as detecting any CTC and clustered CTC postoperatively (Table IV).
Table IV
Multivariate logistic regression analysis of VFL according to the status of postoperative CTC.
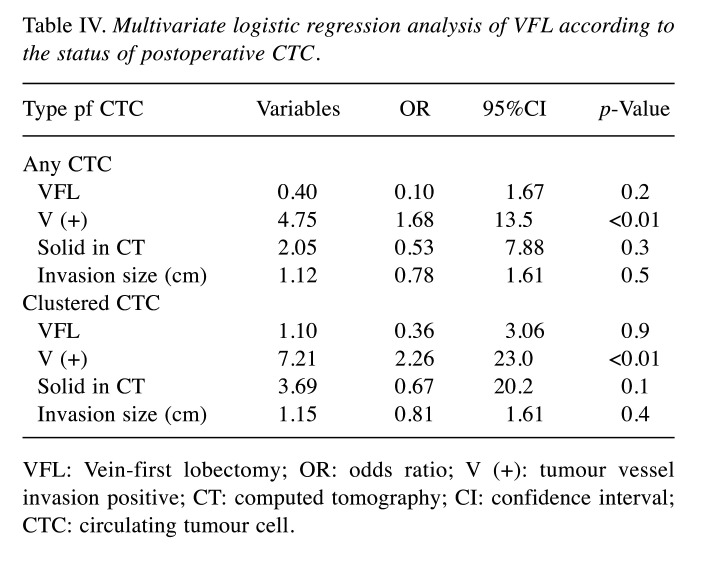
VFL: Vein-first lobectomy; OR: odds ratio; V (+): tumour vessel invasion positive; CT: computed tomography; CI: confidence interval; CTC: circulating tumour cell.
Follow-up results. There were eight cases of death, of which six cases were cancer related. In addition, 25 cancer recurrences were observed, of which 10 cases were distant (5, 2, 2, and 1 at the bone, liver, lung, and brain, respectively) and 15 cases were regional (8, 6, and 1 at the pleural space, mediastinal lymph nodes, and lung, respectively).
The survival curves according to recurrence and the VFL status are shown in Figure 1. The 2-year OS rates were 94.4% and 88.9% in the VFL and no VFL groups, respectively (p=0.4), and the 2-year RFS rates was 78.3% and 72.1% in the VFL and no VFL groups, respectively (p=0.5). In the analysis of OS and RFS according to the presence of postoperative clustered CTC (Figure 2), there were no significant differences in OS between the two groups. However, there were significant differences in RFS between the two groups.
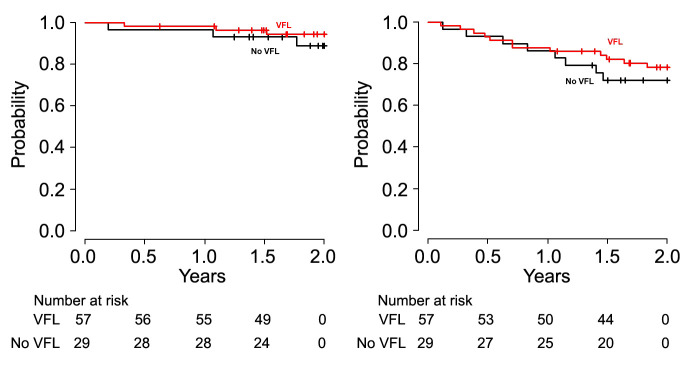
Figure 1Kaplan–Meier curves of overall survival (OS) and recurrence-free survival (RFS) according to the type of pulmonary vein dissection. The 2-year overall survival OS rates were 94.4% and 88.9% in the vein-first lobectomy (VFL) and no-VFL groups, respectively (p=0.4). The 2-year RFS rate were 78.3% and 72.1% in the VFL and no-VFL groups, respectively (p=0.5)

Figure 2Kaplan–Meier curves of overall survival (OS) and recurrence-free survival (RFS) according to the status of postoperative clustered circulating tumour cells. The 2-year OS rates were 97.3% and 88.4% in the patients in whom clustered circulating tumour cells (CTCs) were not detected (N) and detected (P) postoperatively, respectively (p=0.2). The 2-year RFS rates were 93.5% and 54.1% in the N and P groups (p<0.01), respectively.Go to:
Discussion
It has been stated that cancer metastasis occurs mainly via clustered CTC rather than single CTC (4,5,20), although the prognostic impact of VFL and its therapeutic benefits for controlling clustered CTCs are yet to be clarified. In this study, there was no significant difference in both the postoperative types of CTC detection rate and clustered CTC detection rate, and the postoperative mean count of any types of CTC and clustered CTC count between the VFL and no-VFL groups despite postoperative count of any types CTC was significantly greater than the preoperative mean count. Furthermore, the prognosis was also not significantly different, regardless of the presence of clustered CTC after surgery as an indicator of recurrence.
Cancer metastasis occurs when CTCs develop into tumours in the appropriate microenvironment (21), and the presence of postoperative CTCs (22,23), especially clustered CTCs (15,24,25), is a predictor of recurrence. These findings may confirm that surgery causes the release of CTCs and suppressing the number of CTCs may help decrease the risk of postoperative recurrence. In lung cancer surgery, VFL might suppress the release of CTCs, and clinical observation studies have shown that VFL helps reduce recurrence (6-9). However, contrasting findings have also been reported (10-13). A recent study by Wei et al. (6) conducted using propensity score matching has reported better OS and RFS in VFL patients than in no-VFL lobectomy patients, further, VFL suppressed the release of CTCs, as assessed quantitatively using real-time polymerase chain reaction. However, in our current study, there was no significant difference in the CTC status between the VFL and no-VFL groups. This might be because the CTC detection method was different between the study of Wei et al. where clustered CTC was not identified (14), and our study where clustered CTC was detected (17). Moreover, there is a possible difference in the surgical procedure performed in Wei et al.’s study and our present study. In our present study, we performed conventional video-assisted thoracoscopic surgery. Besides, no prognostic difference has been observed in a meta-analysis (13) and another prospective randomized trial (26).
Aside from VFL, other modalities can also cause the release of CTCs Such as bronchoscopic biopsies (27). In addition, preoperative bronchoscopy with fluoroscopic imaging has been reported to be associated with a high incidence of recurrence (28). Further, pulmonary wedge resection for lung cancer has low efficiency for CTC detection after surgery when ring forceps are used without tumour release (29). There is a report revealing significantly better prognosis in patients with early-stage lung cancer who underwent pulmonary wedge resection with ring forceps at the tumour site before lobectomy than in those who did not undergo wedge resection (30).
The efficacy of the vein first technique for suppressing metastasis in cancer is under investigation. A multicentre prospective, randomised trial was conducted to compare the conventional technique and no-touch isolation technique (NTIT) for primary tumour resection in patients with colorectal cancer (JCOG1006) (31), revealing that NTIT was not superior to the conventional technique (32). This result is possibly attributable to the efficacy of adjuvant therapy and the notion that the effectiveness of surgical procedures other than vascular processing may vary among patients. Besides, there is a report revealing the prognostic benefit of surgery for lung cancer is influenced by the surgeon’s experience (33), which is possibly attributable to the potential of shedding CTCs according to the surgical skill.
The present study has some limitations. First, the VFL performed in this setting was not intentional. Second, the CTCs were identified visually, although the accuracy of visual CTC evaluation has been proven in previous studies (34). Furthermore, the method used in this study (ScreenCell®) has the highest recovery rate (35) and is suitable for detecting clustered CTCs, thus yielding more accurate results in evaluating clustered CTCs. Third, the sample size was small, and the patients were only recruited from two centres. Fourth, the observational study design indicates a potential risk of bias. Further prospective studies are needed to verify the implication of VFL.
In conclusion, retrospectively assessed, VFL for lung cancer could not adequately control postoperative clustered CTCs which are a predictor of cancer recurrence.Go to:
Conflicts of Interest
The Authors declare that they have no conflicts of interest in relation to this study.Go to:
Authors’ Contributions
Noriyoshi Sawabata: conception/design of the work; data acquisition, analysis, interpretation; manuscript drafting/revision/ final approval; and agreement to accountability. Noriko Ouji-Sageshima and Takeshi Kawaguchi: data acquisition, analysis, and interpretation; manuscript drafting/revision/final approval; and agreement to accountability. Shigeru Nakane, Daiki Yoshikawa, Keiji Kushibe, and Toshihiro Ito: data interpretation, manuscript revision/final approval, and agreement to accountability.Go to:
Acknowledgements
The Authors thank Dr. Ikuko Torii (Department of Pathology, Hoshigaoka Medical Center) and Dr. Chiho Ohbayashi (Department of Pathology, Nara Medical School of Medicine) for their help in the cytopathological diagnoses. The Authors also thank Editage for English language editing.Go to:
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [PubMed] [CrossRef] [Google Scholar]2. Sawabata N, Miyaoka E, Asamura H, Nakanishi Y, Eguchi K, Mori M, Nomori H, Fujii Y, Okumura M, Yokoi K, Japanese Joint Committee for Lung Cancer Registration Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol. 2011;6(7):1229–1235. doi: 10.1097/JTO.0b013e318219aae2. [PubMed] [CrossRef] [Google Scholar]3. Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med. 2013;19(11):1450–1464. doi: 10.1038/nm.3391. [PubMed] [CrossRef] [Google Scholar]4. Bottos A, Hynes NE. Cancer: Staying together on the road to metastasis. Nature. 2014;514(7522):309–310. doi: 10.1038/514309a. [PubMed] [CrossRef] [Google Scholar]5. Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, Brannigan BW, Kapur R, Stott SL, Shioda T, Ramaswamy S, Ting DT, Lin CP, Toner M, Haber DA, Maheswaran S. Circulating tumor cell clustereds are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–1122. doi: 10.1016/j.cell.2014.07.013. [PMC free article] [PubMed] [CrossRef] [Google Scholar]6. Wei S, Guo C, He J, Tan Q, Mei J, Yang Z, Liu C, Pu Q, Ma L, Yuan Y, Lin F, Zhu Y, Liao H, Wang W, Liu Z, Li Q, Jiang B, Li C, Xia L, Zhao K, Gan F, Cheng J, Wu Z, Wang Y, Lin Y, Kou Y, Che G, Chen L, Li J, Liu L. Effect of vein-first vs artery-first surgical technique on circulating tumor cells and survival in patients with non-small cell lung cancer: a randomized clinical trial and registry-based propensity score matching analysis. JAMA Surg. 2019;154(7):e190972. [PMC free article] [PubMed] [Google Scholar]7. Sumitomo R, Fukui T, Marumo S, Otake Y, Huang CL. Effects of vessel interruption sequence during thoracoscopic lobectomy for non-small cell lung cancer. Gen Thorac Cardiovasc Surg. 2018;66(8):464–470. doi: 10.1007/s11748-018-0943-9. [PubMed] [CrossRef] [Google Scholar]8. Kurusu Y, Yamashita J, Hayashi N, Mita S, Fujino N, Ogawa M. The sequence of vessel ligation affects tumor release into the circulation. J Thorac Cardiovasc Surg. 1998;116(1):107–113. doi: 10.1016/s0022-5223(98)70248-x. [PubMed] [CrossRef] [Google Scholar]9. Huang KL, Deng HY, Fan M, Zheng Q, Lin S, Zhu D, Zhou Q. The sequence of pulmonary vessels ligation during lobectomy for non-small cell lung cancer: A systematic review and meta-analysis. Eur J Surg Oncol. 2021;47(7):1535–1540. doi: 10.1016/j.ejso.2021.02.016. [PubMed] [CrossRef] [Google Scholar]10. Li F, Jiang G, Chen Y, Wang J. Curative effects of different sequences of vessel interruption during the completely thoracoscopic lobectomy on early stage non-small cell lung cancer. Ann Thorac Cardiovasc Surg. 2015;21(6):536–543. doi: 10.5761/atcs.oa.15-00044. [PMC free article] [PubMed] [CrossRef] [Google Scholar]11. He HH, He JX, Hao ZX, Wang W, He JX. Association between different sequences of vessel ligation during video-assisted thoracoscopic lobectomy and survival in patients with non-small cell lung cancer. J Thorac Dis. 2019;11(3):686–693. doi: 10.21037/jtd.2019.02.69. [PMC free article] [PubMed] [CrossRef] [Google Scholar]12. Refaely Y, Sadetzki S, Chetrit A, Simansky DA, Paley M, Modan B, Yellin A. The sequence of vessel interruption during lobectomy for non-small cell lung cancer: is it indeed important. J Thorac Cardiovasc Surg. 2003;125(6):1313–1320. doi: 10.1016/s0022-5223(03)00022-9. [PubMed] [CrossRef] [Google Scholar]13. Toufektzian L, Attia R, Polydorou N, Veres L. Does the sequence of pulmonary vasculature ligation have any oncological impact during an anatomical lung resection for non-small-cell lung cancer. Interact Cardiovasc Thorac Surg. 2015;20(2):260–264. doi: 10.1093/icvts/ivu361. [PubMed] [CrossRef] [Google Scholar]14. Chen X, Zhou F, Li X, Yang G, Zhang L, Ren S, Zhao C, Deng Q, Li W, Gao G, Li A, Zhou C. Folate receptor-positive circulating tumor cell detected by LT-PCR-based method as a diagnostic biomarker for non-small-cell lung cancer. J Thorac Oncol. 2015;10(8):1163–1171. doi: 10.1097/JTO.0000000000000606. [PubMed] [CrossRef] [Google Scholar]15. Manjunath Y, Upparahalli SV, Suvilesh KN, Avella DM, Kimchi ET, Staveley-O’Carroll KF, Li G, Kaifi JT. Circulating tumor cell clustereds are a potential biomarker for detection of non-small cell lung cancer. Lung Cancer. 2019;134:147–150. doi: 10.1016/j.lungcan.2019.06.016. [PubMed] [CrossRef] [Google Scholar]16. Sawabata N, Funaki S, Hyakutake T, Shintani Y, Fujiwara A, Okumura M. Perioperative circulating tumor cells in surgical patients with non-small cell lung cancer: does surgical manipulation dislodge cancer cells thus allowing them to pass into the peripheral blood. Surg Today. 2016;46(12):1402–1409. doi: 10.1007/s00595-016-1318-4. [PubMed] [CrossRef] [Google Scholar]17. Desitter I, Guerrouahen BS, Benali-Furet N, Wechsler J, Jänne PA, Kuang Y, Yanagita M, Wang L, Berkowitz JA, Distel RJ, Cayre YE. A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Res. 2011;31(2):427–441. [PubMed] [Google Scholar]18. Wechsler J, editor. Circulating tumor cells. In: Circulating tumor cells from solid cancers. 1st ed. Paris, Sauramps Medical. 2015;In:pp. 44. [Google Scholar]19. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [PMC free article] [PubMed] [CrossRef] [Google Scholar]20. Gkountela S, Castro-Giner F, Szczerba BM, Vetter M, Landin J, Scherrer R, Krol I, Scheidmann MC, Beisel C, Stirnimann CU, Kurzeder C, Heinzelmann-Schwarz V, Rochlitz C, Weber WP, Aceto N. Circulating tumor cell clustereding shapes DNA methylation to enable metastasis seeding. Cell. 2019;176(1-2):98–112.e14. doi: 10.1016/j.cell.2018.11.046. [PMC free article] [PubMed] [CrossRef] [Google Scholar]21. Eckhardt BL, Francis PA, Parker BS, Anderson RL. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat Rev Drug Discov. 2012;11(6):479–497. doi: 10.1038/nrd2372. [PubMed] [CrossRef] [Google Scholar]22. Sawabata N, Nakamura T, Kawaguchi T, Watanabe T, Ouji NS, Ito T, Taniguchi S. Circulating tumor cells detected only after surgery for non-small cell lung cancer: is it a predictor of recurrence. J Thorac Dis. 2020;12(9):4623–4632. doi: 10.21037/jtd-20-1636. [PMC free article] [PubMed] [CrossRef] [Google Scholar]23. Peach G, Kim C, Zacharakis E, Purkayastha S, Ziprin P. Prognostic significance of circulating tumour cells following surgical resection of colorectal cancers: a systematic review. Br J Cancer. 2010;102(9):1327–1334. doi: 10.1038/sj.bjc.6605651. [PMC free article] [PubMed] [CrossRef] [Google Scholar]24. Tohme S, Simmons RL, Tsung A. Surgery for cancer: A trigger for metastases. Cancer Res. 2017;77(7):1548–1552. doi: 10.1158/0008-5472.CAN-16-1536. [PMC free article] [PubMed] [CrossRef] [Google Scholar]25. Funaki S, Sawabata N, Nakagiri T, Shintani Y, Inoue M, Kadota Y, Minami M, Okumura M. Novel approach for detection of isolated tumor cells in pulmonary vein using negative selection method: morphological classification and clinical implications. Eur J Cardiothorac Surg. 2011;40(2):322–327. doi: 10.1016/j.ejcts.2010.11.029. [PubMed] [CrossRef] [Google Scholar]26. Kozak A, Alchimowicz J, Safranow K, Wójcik J, Kochanowski L, Kubisa B, Pieróg J, Grodzki T. The impact of the sequence of pulmonary vessel ligation during anatomic resection for lung cancer on long-term survival—a prospective randomized trial. Adv Med Sci. 2013;58(1):156–163. doi: 10.2478/v10039-012-0061-3. [PubMed] [CrossRef] [Google Scholar]27. Sawabata N, Kitamura T, Nitta Y, Taketa T, Ohno T, Fukumori T, Hyakutake T, Nakamura T. Lung cancer biopsy dislodges tumor cells into circulating blood. Journal of Cancer Metastasis and Treatment. 2017;3(1):16. doi: 10.20517/2394-4722.2016.67. [CrossRef] [Google Scholar]28. Yasukawa M, Sawabata N, Kawaguchi T, Kawai N, Taniguchi S. Clinical implications of transbronchial biopsy for surgically-resected non-small cell lung cancer. In Vivo. 2018;32(3):691–698. doi: 10.21873/invivo.11295. [PMC free article] [PubMed] [CrossRef] [Google Scholar]29. Sawabata N, Hyakutaka T, Kawaguchi T, Yasukawa M, Kawai N, Tojo T, Taniguchi S. A no-touch technique for pulmonary wedge resection of lung cancer. Gen Thorac Cardiovasc Surg. 2018;66(3):161–167. doi: 10.1007/s11748-017-0863-0. [PubMed] [CrossRef] [Google Scholar]30. Yasukawa M, Sawabata N, Kawaguchi T, Taniguchi S. Wedge resection of tumor before lobectomy for lung cancer could be a no-touch isolation technique. In Vivo. 2020;34(2):779–785. doi: 10.21873/invivo.11838. [PMC free article] [PubMed] [CrossRef] [Google Scholar]31. Takii Y, Shimada Y, Moriya Y, Nakamura K, Katayama H, Kimura A, Shibata T, Fukuda H, Colorectal Cancer Study Group (CCSG) of Japan Clinical Oncology Group A randomized controlled trial of the conventional technique versus the no-touch isolation technique for primary tumor resection in patients with colorectal cancer: Japan Clinical Oncology Group Study JCOG1006. Jpn J Clin Oncol. 2014;44(1):97–100. doi: 10.1093/jjco/hyt156. [PMC free article] [PubMed] [CrossRef] [Google Scholar]32. Takii Y, Mizusawa J, Kanemitsu Y, Komori K, Shiozawa M, Ohue M, Nishimura Y, Ikeda S, Takiguchi N, Kobatake T, Ike H, Sato T, Tomita N, Ota M, Masaki T. A randomized controlled trial of the conventional technique versus the no-touch isolation technique for primary tumor resection in patients with colon cancer: Primary analysis of Japan Clinical Oncology Group study JCOG1006. Journal of Clinical Oncology. 2020;37(15_suppl):3515–3515. doi: 10.1200/JCO.2019.37.15_suppl.3515. [CrossRef] [Google Scholar]33. Scheel PJ 3rd, Crabtree TD, Bell JM, Frederiksen C, Broderick SR, Krupnick AS, Kreisel D, Patterson GA, Meyers BF, Puri V. Does surgeon experience affect outcomes in pathologic stage I lung cancer. J Thorac Cardiovasc Surg. 2015;149(4):998–1004.e1. doi: 10.1016/j.jtcvs.2014.12.032. [PMC free article] [PubMed] [CrossRef] [Google Scholar]34. Sawabata N, Susaki Y, Nakamura T, Kawaguchi T, Yasukawa M, Taniguchi S. Clustered circulating tumor cells in surgical cases of lung cancer. Gen Thorac Cardiovasc Surg. 2020;68(9):975–983. doi: 10.1007/s11748-020-01308-3. [PubMed] [CrossRef] [Google Scholar]35. Drucker A, Teh EM, Kostyleva R, Rayson D, Douglas S, Pinto DM. Comparative performance of different methods for circulating tumor cell enrichment in metastatic breast cancer patients. PLoS One. 2020;15(8):e0237308. doi: 10.1371/journal.pone.0237308. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
原文链接:http://www.xxwk.net/archives/2259